|
| Indian Ocean Dipole and El Nino Southern Oscillation |
Both the ENSO and the Indian Ocean Dipole are ocean atmospheric systems that consist of the relative sea-surface temperature and the global wind systems, with the ENSO a system which affects predominantly the pacific ocean and the Indian Ocean Dipole affecting the Indian ocean. These Ocean temperature and wind systems are an important control system on the nutrient cycle within the oceans but also for agriculture as they affect rainfall patterns and intensity and as such we will be looking at the effects of both these cycles within the Indian Monsoons.
ENSO: When People think of monsoons they often think of the Indian Monsoons which are also closely related to torrential rainfall within Indian. However, this is completely true as monsoons are common on other parts of the globe and monsoons are also associated with periods of drought as the monsoons as a system are related to the seasonal change in wind direction and rainfall patterns driven by shifts in temperature differences between the ocean and land causing pressure differentials as depicted in the diagram below. It is this temperature dependency of the monsoons on oceans that the Indian Ocean Dipole and ENSO become important systems to understand.
 |
| Indian Seasonal Monsoons. |
ENSO is a climatic pattern involving changes in the temperature of the waters in the central and eastern tropical Pacific Ocean. It involves the meeting of the Northern and Southern Hadley cells at the ITCZ, in which surface Easterly winds are weak causing the Western boundary of the pacific ocean surface to rise by 0.5-0.75m compared to the Eastern boundary during the normal Walker Cycle. However, under El Nino conditions the Easterly winds weaken further causing the Western boundary seal level to drop causing the warm water to be evenly spread throughout the pacific ocean increasing the depth of the thermocline and causing rain to fall over the central pacific ocean as depicted below.
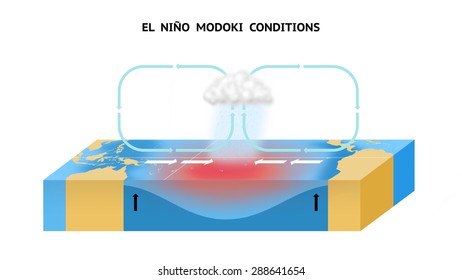 |
| El Nino Conditions over the pacific ocean. |
Indian Ocean Dipole: The Indian Ocean Dipole (IOD) is similar to the ENSO because it speaks about the difference in sea surface temperatures of the Eastern and Western Boundaries of the Indian Ocean. The IOD has three phases, just like the ENSO, with the three phases being neutral, positive and negative. Neutral Conditions are when sea surface temperatures over the whole Indian ocean are relatively normal but during the positive phase of the IOD the Westerly winds weaken allowing warmer waters to concentrate on the tropical Eastern boundary of Africa increasing land rainfall within this region. The IOD is also an important driver of the Australian climate having a strong impact on the agriculture of the region and more recently the bush fires which have occurred as this years positive IOD hit 2 degrees Celsius exaggerating the dry conditions experienced in Australia.
Now that we have a basic understanding of how the Indian Monsoons work, the ENSO and the IOD, how do the two ocean atmospheric systems interact in influence the Indian Monsoons. A study conducted by Cherchi, showed that rainfall decreased during the Indian summer when the El Nino and positive IOD occurred concurrently with a study by Ashok, confirming this trend. However, both studies also showed how the different phases of both system when occurring together but not in synergy either have amplifying or reducing effects on the monsoon rains, but it was also noted by Ashok, that the IOD had weakened the ENSO-monsoon relationship.
In Conclusion each of the different ocean-atmospheric system when looked in isolation have differing effects but when occurring together have both positive and negative effects on each other depending on the feedback mechanisms of the climates they influence.
Reference List:
Ashok, K., Guan, Z., Saji, N.H. and Yamagata, T., 2004. Individual and combined influences of ENSO and the Indian Ocean dipole on the Indian summer monsoon. Journal of Climate, 17(16), pp.3141-3155.
Cherchi, A. and Navarra, A., 2013. Influence of ENSO and of the Indian Ocean Dipole on the Indian summer monsoon variability. Climate dynamics, 41(1), pp.81-103.
Hashizume, M., Chaves, L.F. and Minakawa, N., 2012. Indian Ocean Dipole drives malaria resurgence in East African highlands. Scientific reports, 2(1), pp.1-6.